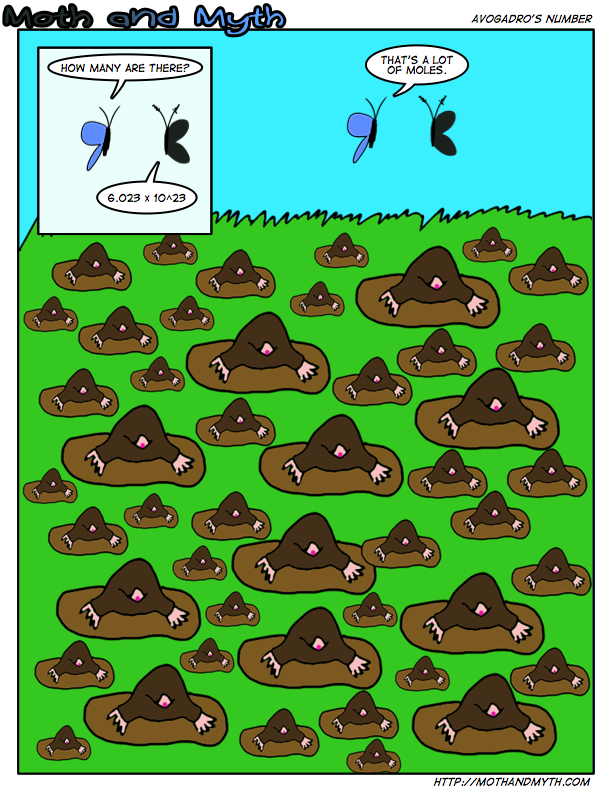
A mol of moles! Though, really, since I wrote this, the head honchos changed Avogadro’s number to 6.022 x10^23, instead of 6.022×10^23. Oh well. You will have to suffer with what my chemistry textbooks taught me.
I never really understood what that unit was about for the longest time, since this is how it was inevitably described to me:
“A mol is whatever you want it to be!”
“So… can it be a hot fudge sundae?”
“You can have a mol of that, sure! But, really, it’s the amount of carbon atoms in a gram.”
“…um… what about my hot fudge sundae?”
“Sure, it can be that as well!”
WHAT?
So, for those suffering from my confusion, the simple answer is this:
A mol is a unit to measure atomic quantity, with it being based on the number of carbon atoms in a gram. Except it’s weirder than that but… just ignore that bit.
Lolololollooolll I loove this!